In many manufacturing sectors, parts cleaning is critical to quality reassurance. Insufficiently cleaned parts can affect the quality of many subsequent industrial processes such as coating, welding, bonding and assembling. This can have a detrimental effect on the functionalities of the parts. Particularly in highest-value manufacturing sectors such as aviation and defense, medical technology and motor vehicles, cleaning quality is not only synonymous with quality, but reliability and even safety.
So, when it comes to industrial parts cleaning, many people instinctively ask:
What is better – aqueous cleaning or solvent cleaning?
The answer is: it depends.
The truth is, both are effective cleaning methods. Since aqueous and solvents work in a completely different way, depending on the cleanliness requirements, the components to be cleaned and the contaminations, one may be more effective than the other in certain situations.
In fact, the question has never been about which one is better. It is rather: what are your requirements, goals and expectations and how can these demands be best fulfilled?
Remember you are not merely trying to do critical cleaning. You want to do the job in the most economically viable and safest way while meeting regulatory requirements and protecting the environment. Making the right decision therefore depends on a myriad of factors – technical, economic, safety and health. The optimal decision should work to balance those needs.
How do aqueous cleaning and solvent cleaning work?
In order to identify the right cleaning solution for your application, first, you need to understand how these two approaches differ in their working.

Aqueous cleaning
Aqueous cleaning relies on chemical additives and agitation in most cases. Detergents, surfactants, emulsifiers or buffers are added to the water to facilitate and enhance its cleaning performance for the removal of contaminants. Aqueous cleaning can also be aided by heat, agitation and time.
Mostly, multiple wash stations are followed by rinsing baths to remove any residues.
Parts are then dried with heaters or blowers.
Aqueous cleaning solutions are usually concentrates which are added to the water. They are often “soil specific”, which means the detergents/chemicals have to match the contaminants and the metal types to be cleaned.
As soils and contaminations are emulsified and flooded off the surface which remain in the water (unless specific procedures are performed to purify the water), the effectiveness of aqueous cleaning will mainly depend on the quality of the cleaning baths, as well as the number and quality of the rinsing baths with demineralized water.
The higher the required quality of cleaning, the investment and space requirements for the aqueous systems will increase accordingly.
The water consumed in the aqueous cleaning process, which is now contaminated with oil, will be disposed entirely as waste water, unless it can be recycled in appropriate facilities.

Solvent cleaning
Solvents dissolve contaminations in the cleaning solution. In other words, soils are diluted in the solvents. Metal parts are immersed in a solvent (or sprayed) that is continuously conditioned through filtration and distillation. By employing heat, agitation, and prolonged time, the effectiveness of solvent cleaning can be enhanced further still.
In solvent cleaning, the parts undergo a process known as vapor cleaning. The process ensures that pure solvent vapor accesses every part of the surface including minuscule holes. The vapor then condenses on the cooler parts, eliminating any leftover oil film. The cleaned parts are then removed dry following a (vacuum) drying process.
One of the most important features of solvents is their universal compatibility. They work well with various metal types and are generally effective against a broad array of contaminants.
Solvents have good drying behavior and they can penetrate easily into tight spaces. But such properties can also bring inherent risks such as air emissions or ground penetration.
In the past, solvent losses into the air from open top machine used to represent a major portion of organic pollution. The use of modern closed (vacuum) machine technology, in combination with a closed loop solvent delivery system, is therefore key to ensuring a safe and virtually emission-free solvent cleaning process.
What are the common solvent types?
| Non-halogenated solvents | |
|---|---|
| Modified alcohols | Standard hydrocarbons |
| Halogenated solvents | ||
|---|---|---|
| Perchloroethylene | Methylene chloride | Fluorinated solvents |
Key differences in working mechanism between AQUEOUS CLEANING and solvent cleaning
| Aqueous cleaning | Solvent cleaning | |
|---|---|---|
| Working principle | By dissolving and emulsifying contaminations off surfaces. | By dissolving soils in solvents |
| Additives | Additives[1] such as detergents, surfactants, emulsifiers, chelating agents, saponifiers, acids, alkalis or builders are added to water to enhance cleaning performance. | Not required (apart from small amounts of stabilizers if necessary) |
| Process steps | Often multi-stage process with multiple wash tanks followed by rinse station(s) and additional drying – therefore product carry-over | Single process taking place within an enclosed solvent degreasing unit – no product carry-over |
| Cleaner concentration | Cleaner concentration will change due to evaporation, consumption and carry-over, and therefore must be closely monitored. Regular re-dosing or replacement might be necessary. | Cleaner concentration remains consistent throughout |
| Cycle time | Longer | Shorter |
| Floor space requirement | Larger (usually horizontal set-up) | Smaller |
| Drying | Additional drying step with heater or blower to remove leftover residue | Normally no additional drying step as parts come out dry of the cleaning machine |
| Water consumption | Yes | No |
| Metal compatibility | Detergents/chemicals need to match the metal types | Universal compatibility with metals |
| Contaminant type | More suitable for polar, inorganic contaminants | More suitable for nonpolar, organic contaminants |
| Waste treatment | Proper treatment and disposal of waste stream necessary (wastewater/oils (contaminants)) | Waste stream mainly consisting of removed metal treatment fluids and a small amount of used solvent in the distillation unit needs to be disposed properly |
1Some additives can be very aggressive to different metals.
How should you choose the right cleaning agent ? 10 key questions to ask.
As mentioned before, depending on the requirements and the specific context, both aqueous and solvent solutions can achieve excellent cleaning results. So how should you go about choosing the right cleaning agent for your metal cleaning? We’ve highlighted 10 key questions for you to consider.
Knowing your required cleaning quality requirements is fundamental to choosing the right cleaning agent.
To achieve optimal cleaning results, you need to consider two different kinds of soils:
1) particle contaminations, including their size, numbers and types and
2) the sort of filmy contaminations.
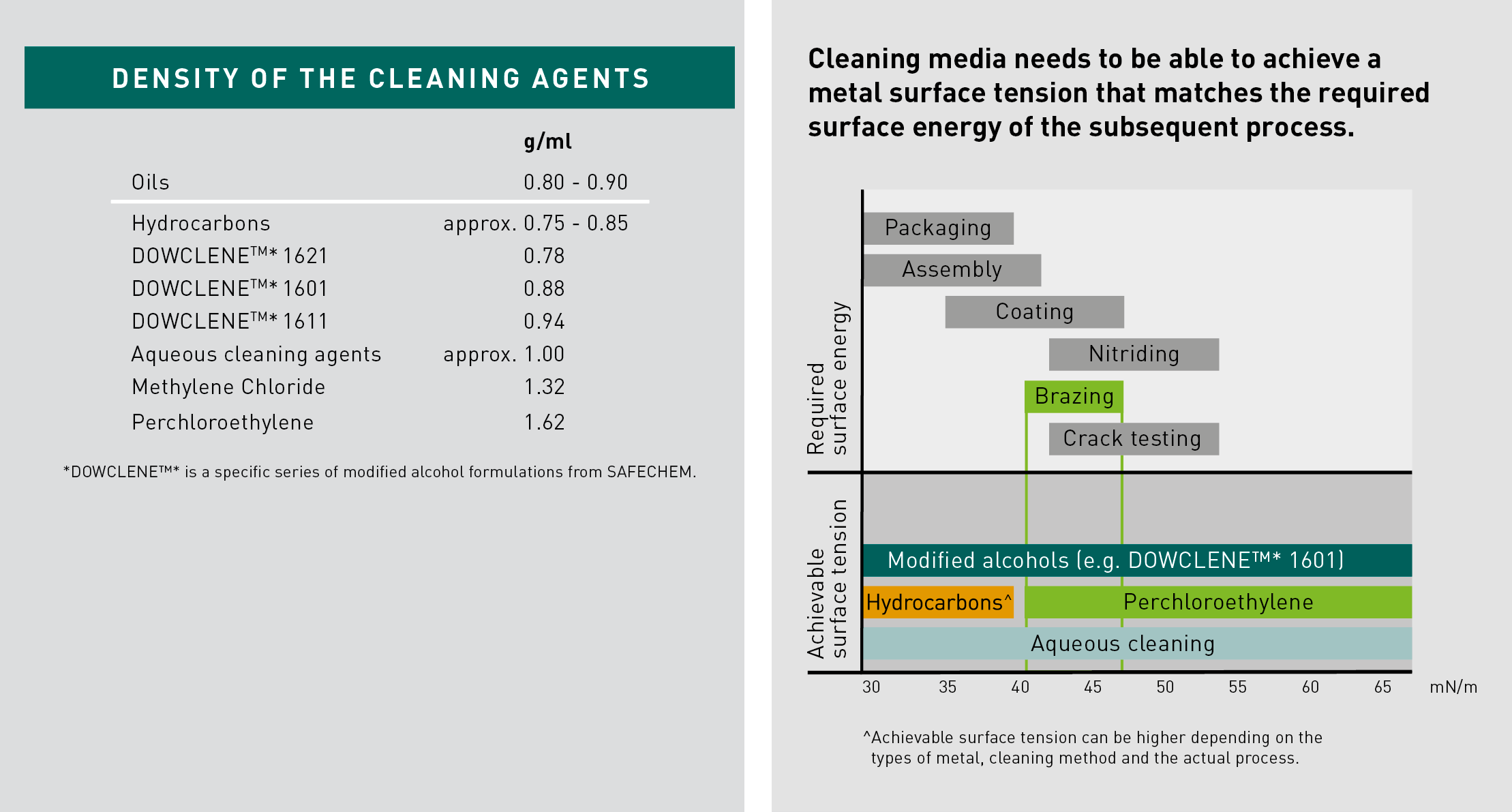
“Heavier” cleaning agents are effective in removing particulates that cannot be readily dissolved. They can literally “push” or ”float away” particulates and lift them off the surface. Aqueous cleaning agents have a density of approx. 1g/ml. Solvents may have a higher or lower density.
Filmy contaminations can affect the required surface energy of the metal surface, which varies depending on the industrial applications. For example, nitriding or crack detection requires a higher surface energy than standard coating or assembling. The required surface energy should therefore match the ability of the cleaning agent.
For precision cleaning where required surface energy can range anywhere from 38 mN/m to approx. 60 mN/m, both aqueous-based cleaning and solvents are capable of fulfilling the requirement.
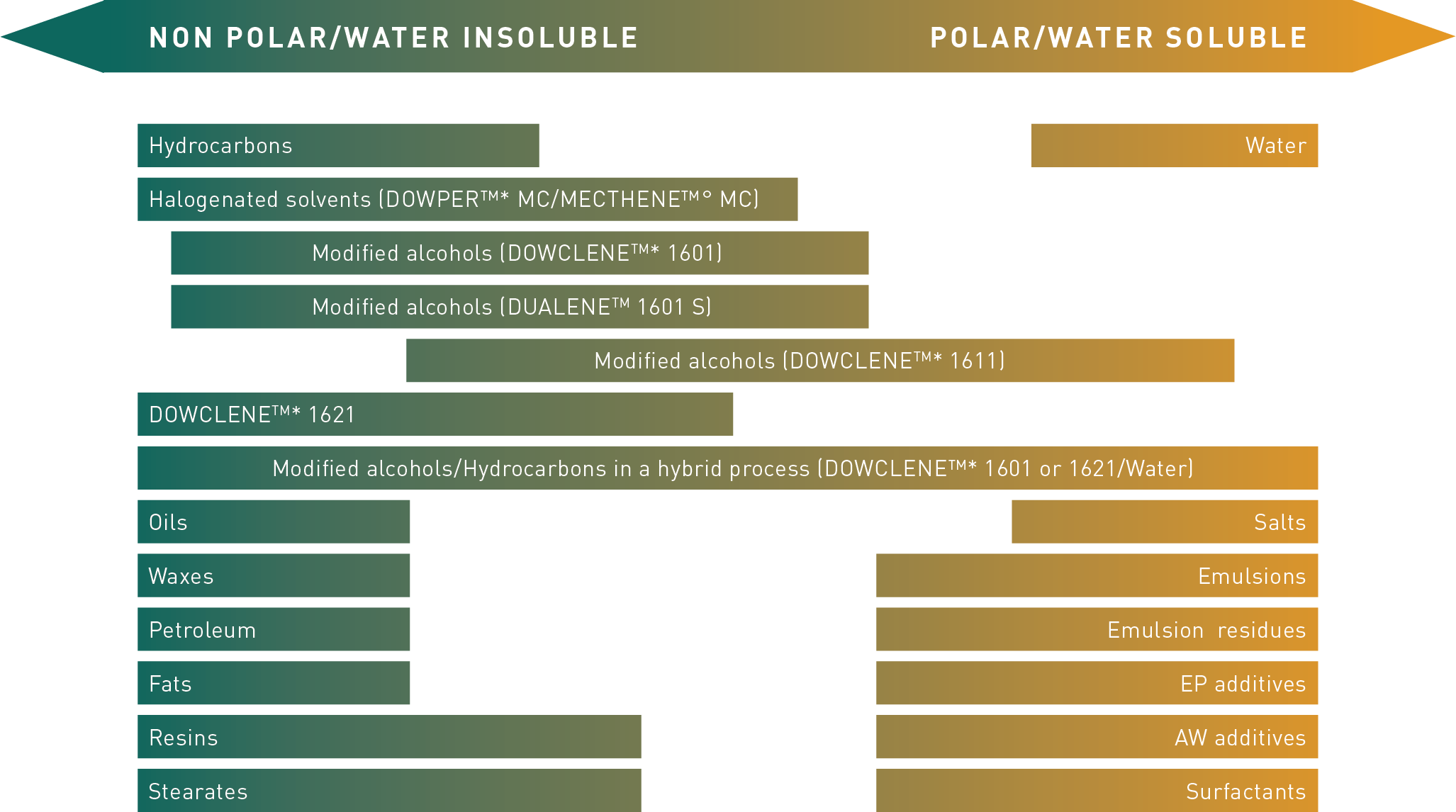
Effective cleaning is based on the principle “Equal dissolves equal”. To achieve optimal metal cleaning results, the cleaning agent should be chemically similar to the contaminant.
There are polar (inorganic) and non-polar (organic) contaminations. For water-based (polar) types of contaminations such as coolant and lubricant emulsions as well as solids like chips, salt, residues of polishing pastes and particles, aqueous cleaners are typically the first choice.
These are available in neutral pH, alkaline and acidic formulations, and a huge variety of surfactants and other ingredients.
When removing mineral oil based, non-polar contaminations, such as machining oils, greases and waxes, solvent will commonly be the preferred cleaning agent.
Nowadays modified alcohols are also available; due to their both non-polar and polar properties, they are able to clean non-polar contaminations as well as certain polar contaminations. This type of solvents has a much broader application range compared to traditional non-polar solvents.
In water-based cleaning, cleaning agents which can be acidic, neutral or alkaline, is usually matched to specific metal types. Simultaneous cleaning of different metals can therefore be problematic and this can result in compatibility issues and in worst case: corrosion.
Sometimes reactive additives are added to water to modify metal surface in processes such as edging and pickling. However, at times such reactive additives can also act aggressively on certain metal surfaces where it is not intended.
Solvents generally have broad material compatibility, which means they are compatible with all kinds of metal, when used properly, making it a suitable option for universal cleaning.
If the component parts are tiny or have complex geometry or small crevices, solvent is often recommended due to its lower surface tension and viscosity which makes it easy to wet into and evaporate out of tight spaces.
With water-based cleaning, even tiny traces of residue moisture could give rise to issues such as challenges in subsequent production processes, corrosion, or growth of bacteria and related bioburden issues.
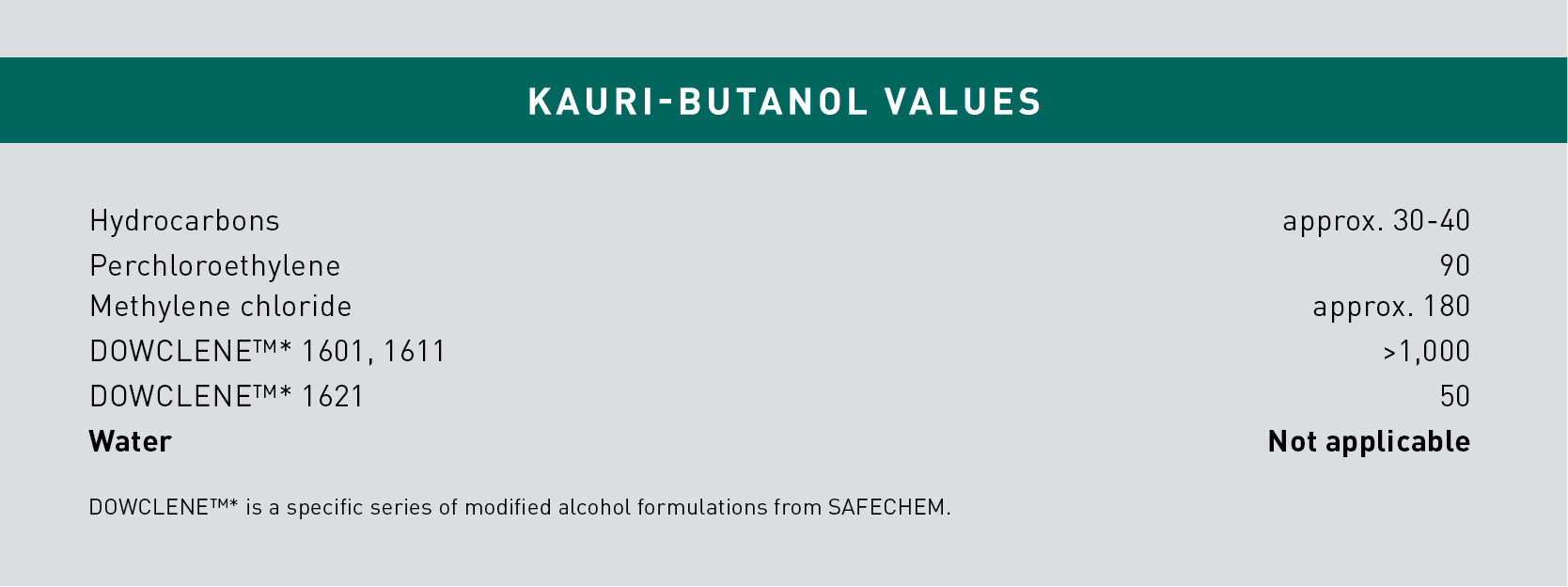
One commonly used indicator for rating the cleaning strength of a non-aqueous solution is the Kauri-Butanol (KB) value. It can range anywhere from very mild (10) to very strong (+1000). The higher the value, the better the relative cleaning power.
Nevertheless, a high KB value can also cause materials compatibility issues if the strength of the cleaning is proving too aggressive for certain materials. Please note that KB value is not applicable for water.
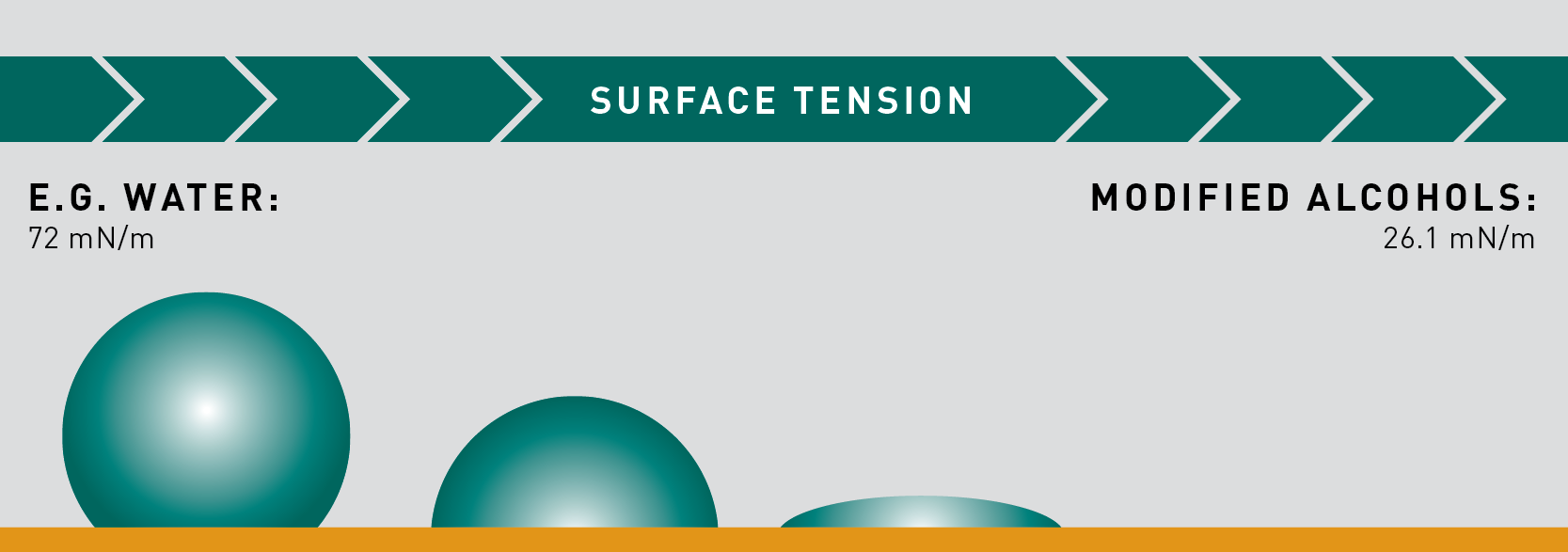
When cleaning geometrically complex components, both surface tension and viscosity of the cleaning agent should be considered.
Surface tension is defined as the attraction of molecules on a liquid’s surface. The higher the surface tension, the higher the tendency of liquid surfaces to shrink into the minimum surface areas possible.
Given that solvents have lower surface tension than water, they have greater ability to wet a surface (as well as evaporate out of tiny places) and can thus more effectively permeate tight clearance areas to remove soils.
Viscosity measures a liquid’s resistance to flow. The lower the viscosity, the better a liquid can flow around objects and get into (and out of) tight spaces more easily.

In water-based cleaning, significant energy is required for pre-treating and heating up cleaning water, operating high-pressure pumps, drying parts as well as treating wastewater.
Compared to the higher volatility of solvents, water is a slow-drying cleaner and requires more elaborated drying procedures aided by heaters or blowers. Water requires 10 times more of the latent heat of vaporization (2259 J/g) than that of solvents, which lie typically between 200-300 J/g.
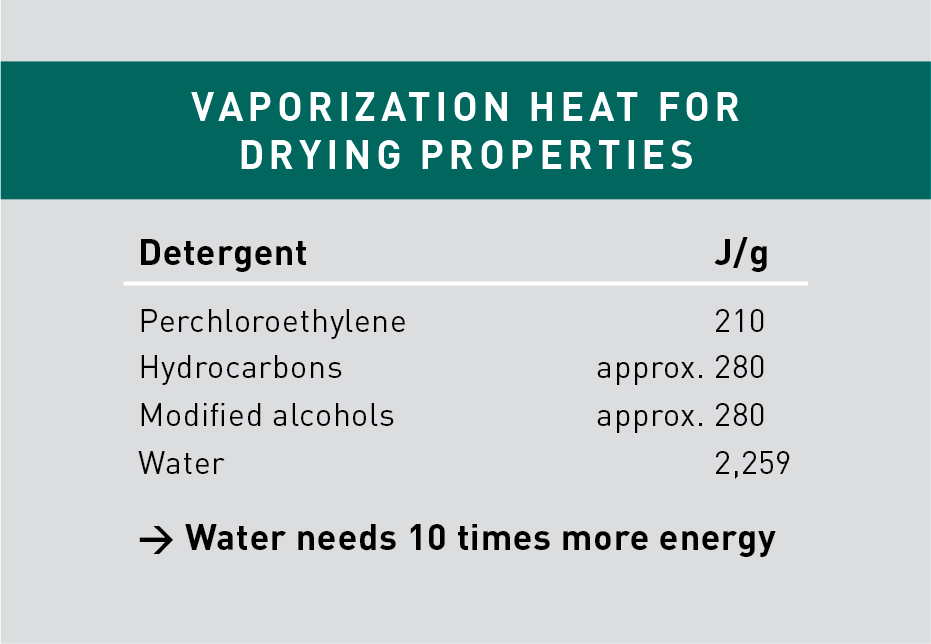
Solvent vapor degreasing also requires energy to keep the entire operation under vacuum condition. As operating temperatures are reduced accordingly in a vacuum, the boiling point of solvent is also reduced, making evaporation quick and effective.
Vapor degreasing systems usually work vertically, compared to aqueous cleaning which often operates horizontally (a typical aqueous batch system may have one wash tank and 2-5 rinse tanks, with the number of rinse tanks depending upon the required cleaning quality). Water-based cleaning therefore tends to require a larger footprint (and more electricity to run). The humidity and heat generated by the aqueous cleaning system also needs to be counteracted by the air conditioning system, another energy cost factor that should be considered.
The capacity and cycle times of your parts cleaning method should match your throughput requirements.
Given that the purchase of any cleaning equipment is a long-term investment, you need to bear in mind your future growth. Because of the need for washing, rinsing and drying in aqueous cleaning, degreasing with solvent typically has shorter cleaning cycles in comparison.
Depending on the required cleanliness standards, additional mechanical actions or temperature treatment might be required in aqueous cleaning which can impact cycle time and throughput rate.
That said, with solvent cleaning, metal parts are cleaned in batches whereas inline aqueous cleaning allows the cleaning process to be integrated into the production line.
Since dirts and soils are emulsified and rinsed off in aqueous cleaning, aqueous baths that are not treated have to be replaced frequently.
In contrast, solvent can be recycled indefinitely via the distillation unit, which is built within the vapor degreasing system or as a separate additional system.
The continuous distillation and re-purification of the solvent significantly increases solvent lifespan and cuts down on waste volume. This again leads to reduced solvent consumption and solvent costs (therefore a lower cost per-cleaned-part).
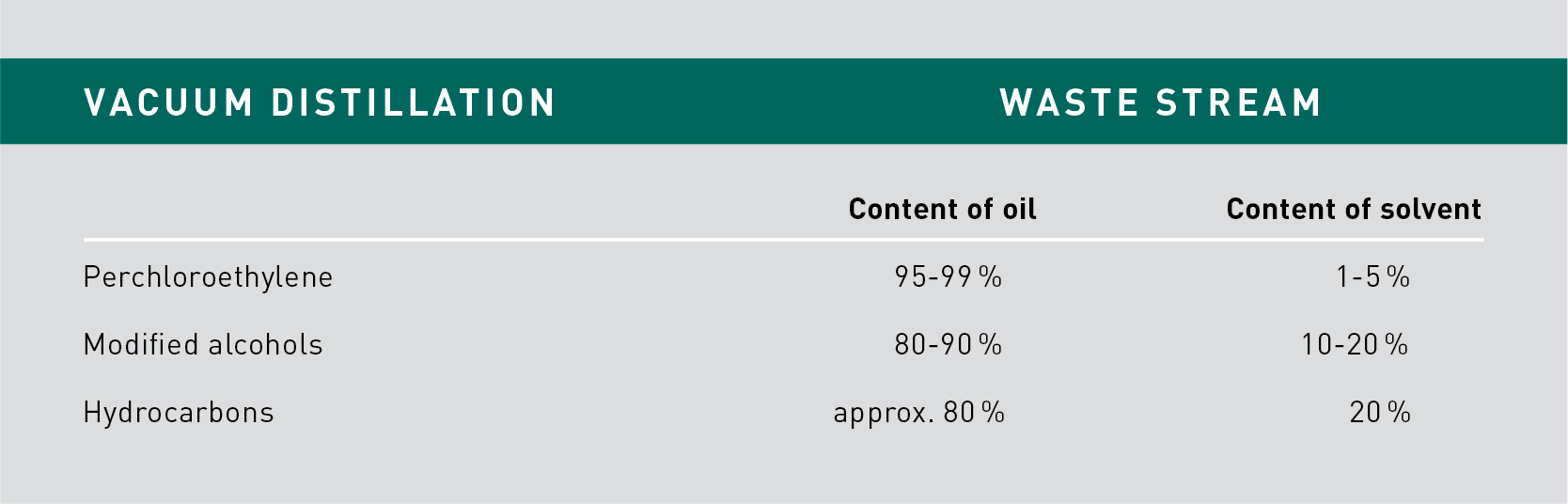
If there is a large amount of oils to be cleaned off, the opportunity to separate solvent from oils (via distillation) can be a particular advantage, since solvent can be effectively recovered, leaving only very little solvent residues in the waste.
Generally, the higher the difference in boiling point/range between solvent and the oil, the better they can be separated in the distillation.
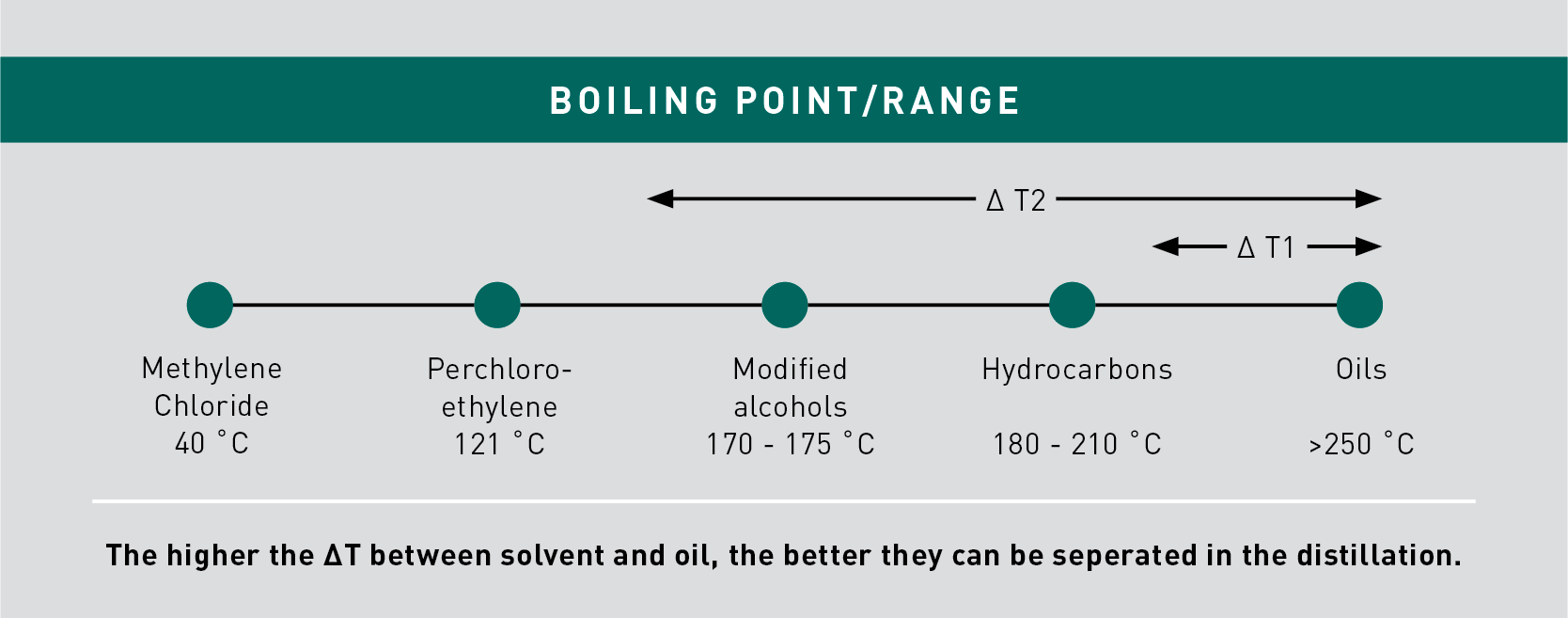
As an example, given that the boiling point of Perchloroethylene is significantly lower than that of oils (>250 °C), the solvent can be most effectively separated from oils in the distillation sump. Solvent content in the waste can be as low as just 1% (or even lower).
Regular bath monitoring and maintenance is indispensable for achieving optimal cleaning results and longer bath lifetimes for both water-based and solvent cleaning.
In aqueous system, chemicals such as builders, surfactants, emulsifiers, sequestering/chelating agents and buffers are added to enhance the cleaning profile.
The concentration of these chemicals must be constantly monitored and adjusted to secure a stable process. In many cases, more than one or even multiple tests will be required every week.
Aqueous cleaning can be prone to bioburden issues. To avoid growth of bacteria, the use of biocides might be necessary.
Parts cleaning with solvent requires no additional chemicals (beside stabilizers), so the chemistry in the vapor degreaser remains consistent and requires little attention to ensure a constant cleaning performance.
Nevertheless, as used solvent is constantly re-purified and recycled via distillation – meaning more work demand is made on less solvent input – the stress on solvents could increase.
To extend solvent lifespan, on-site solvent monitoring and control can be conducted once a week in no more than 20 minutes. Where necessary, stabilizer concentrates can be added to counteract evolving acidity, prevent discoloration or metal catalytic effects among others, while enabling safe pH value and reliable processes. It is recommended to undertake a full solvent analysis twice a year to ensure solvent quality.
Basing your investment decision on the sole cost of the equipment or per-liter cost of the cleaning agent is a guaranteed recipe for distorted conclusion. To access the true economic costs of cleaning, you must look at both the acquisition costs and the ongoing costs.
Acquisition costs entail the price of the machine, installation, and retrofits. Operating costs include consumable solvents or detergents/additives, utility bills for electricity/water, process control and bath maintenance, floor space, waste disposal charges, not to mention labor costs in terms of hours and efforts in operation and process monitoring.
By taking into account all the cost parameters, a total cost-per-part cleaned can be devised, which is a much more meaningful and robust indicator of the cost efficiency of your parts cleaning system.
Comparison of Cost Factors
| Aqueous cleaning | Solvent cleaning | |
|---|---|---|
| Initial capital expenditure for machine and cleaning agents | Often lower for equipment set-up and cleaning agents (To enhance cleaning performance and machine efficiency, the addition of ultrasonics, evaporators for water, treatment plants for wastes etc can increase initial investment accordingly). | Often higher for standard solvent machine technology and initial first fill chemicals. |
| Water consumption | Buying and pre-treatment of water, plus disposal of wastewater.. | Zero water consumption. |
| Energy consumption | Higher due to increased temperature for optimal cleaning, in addition to extra energy for drying cleaned parts. | Lower (no need for additional heat during vacuum drying normally). |
| Cleaner replacement | More frequent replenishment and bath exchanges as aqueous cleaners get consumed in the process. | Very low solvent replacement volumes, and many fewer solvent bath exchanges, due to continuous solvent recycling via in-built distillation. |
| Process control and bath maintenance costs | Higher due to the need to constantly monitor and adjust the concentration of the chemicals (e.g. builder and surfactants) to secure a stable process. Potential bioburden issues add another layer of process control and necessitate the use of biocides. | Lower as no additional chemicals (beside stabilizers when necessary) are required, so the chemistry in the vapor degreaser remains consistent and requires little attention to ensure a constant cleaning performance. |
| Floor space costs | Higher due to multi-station horizontal machine set up and several cleaning/rinsing bathes | Lower due to vertical machine set-up and vapor degreasing last cleaning step |
| Disposal costs | Amount of oils remove plus the contaminated water from the cleaning and the rinsing tanks | Amount of oils removed plus up to 1-25% of used solvent (depending on the solvent in use) |
Whether it is aqueous or solvent cleaning, both processes require prudence and diligence from users in ensuring worker safety, environmental protection and regulatory compliance.
In aqueous cleaning, effluent treatment is a key issue. Although water tends to be associated with sustainability or “green”, it is a finite and precious resource. Certain water-based cleaners are also classified as more dangerous than specific solvent cleaners in their toxicological profile. Proper treatment of wastewater is therefore of crucial importance.
Even if the water-based cleaners are biodegradable, given that soils generally are not, the wastewater still needs to be treated and disposed properly, especially since it can contain anything from cleaning additives to oil, lubricants, fluxes, particulates etc.
Companies who fail to do so not only risk high penalties, worse still, their negligence can severely impact the ecological profile and the local communities.
Similarly, while solvent cleaning does not consume water, it still demands a well-defined solvent risk management approach across storage, handling and external recycling of used solvent/disposal of waste oils.
As solvents can give off volatile organic compounds (VOCs), the use of closed cleaning machines with inherent distillation unit, along with a closed-loop solvent delivery system, can significantly reduce air emissions and waste to an absolute minimum (solvent consumption is optimized simultaneously since less solvent is lost to the air and less waste is generated).
Modern closed cleaning machines are now engineered to avoid any interface between operator and solvent. In most cases the working chamber can only be opened when solvent volume falls below a defined limit. In addition, the vacuum operation model in closed machines also ensures that flammable solvents are safely handled.
Regardless of which cleaning method is chosen, there are risks involved. It is therefore vital to put in place adequate risk management policies where key aspects such as toxicological information, corrosivity, exposure risks, and flammability etc are carefully examined and considered.

Summary
Advantages of aqueous cleaning vs solvent cleaning
| Aqueous cleaning | Solvent cleaning |
|---|---|
|
|
*Non-flammable solvents such as Perchloroethylene also exist.
COMPARISON OF PROPERTIES OF DIFFERENT CLEANING AGENTS
| Boiling Point(°C) | Evaporation Energy(j/g) | Flash Point(°C) | Surface Tension(mN/m) | Density(g/ml) | KB-value | Solubility in water(%) | Viscosity(mPa·s) | |
|---|---|---|---|---|---|---|---|---|
| Methylene Chloride | 40 | 329 | - | 28,1 | 1,32 | 136 | 2 | 0,42 |
| Perchloroethylene | 121 | 209 | - | 32,3 | 1,62 | 92 | 0,01 | 0,88 |
| DOWCLENETM* 1601 / DUALENETM1601 S(Modified Alcohols) | 170-175 | 280 | 63 | 26,1 | 0,88 | 1000 approx. | 6,3 | 3,2 |
| Standard Hydrocarbon | 175-195 | 280 | 59 | 24 | 0,78 | <30 | 0,00001 | 1,2 |
| Water | 100 | 2260 | - | 72,8 | 1 | - | - | 1 |
| Fluorinated solvent trans-dce blend | 45 | 220 | - | 20 approx. | 1.25 approx. | 124 (Between 10-14 if without T-DCE) | 0.004-0.012 | 0,42 |
| Trans - 1,2 Dichloroethylene | 47 | 336 | 2 | 25 | 1,27 | 117 | 0,06 | 0,41 |
Modified alcohols and standard hydrocarbons – as all non-halogenated solvents – do have a flash point, therefore they must be deployed in closed cleaning machines under vacuum when operating with high temperatures.
Nevertheless, even when handling non-flammable solvents, solvent cleaning in closed vapor degreasing machines is still strongly advisable because it ensures the highest standard of worker safety, environmental protection as well as sustainability in terms of reduced solvent consumption and minimized wastage.
Key takeaways
Metal cleaning is more than simply achieving the desired cleanliness in a consistent and reliable manner. An effective cleaning process should fulfill the cleaning needs in the most economical way, where risks are properly managed and kept to a minimum, employee welfare and safety is looked after, and sustainability and regulatory compliance are well demonstrated.
Every industrial process is unique within its own specific context, so are the goals and the requirements. A cleaner that works excellently in one context may bring insufficient results in another, depending on the complexity of the parts, the contaminations, and the throughput requirements. Because of the many intertwined factors in play, it would be oversimplified to make an overall credible judgment of the superiority of one cleaning media over another.
No cleaning system is without environmental impact. They only manifest in different ways and we need to look at the big picture as a whole when considering various factors, such as the use of chemicals, water consumption, energy usage, recycling potential, waste handling and risk management approach etc. Ultimately, the goal should be to minimize emissions, pollution and waste as much as possible while still meeting the cleaning requirements.
The performance of the solvent itself is just as crucial as the process and system within which it is used. Great technological advances in closed-loop solvent vapor degreaser are leading to environmentally sound and responsible cleaning options. Taking into account energy usage, solvent recyclability, waste treatment and disposal, as well as chemical impact on the environment, solvents when used in combination with modern closed cleaning equipment, can have an even better environmental profile than many other cleaning methods.
The investment in a proper cleaning set-up is a considerable financial commitment. It is also a decision with long-term impact. More than just a necessity, parts cleaning – when done properly – can deliver much more value than the mere technical function it fulfills, in terms of driving operational efficiency, savings and sustainability performance.
Final words
The selection of the right cleaning solution requires objective and considered evaluation of the substrates, contaminants, process requirements and constraints, throughput and future projections – in addition to costs, safety, environmental impact and regulations.
In particular, the perfect interplay between machine technology and the cleaning medium will be key to achieving required cleanliness in a consistent, reliable and cost-effective manner. It is therefore critical to conduct compatibility and efficiency testing in commercial cleaning systems, as well as in-depth consultation with chemicals suppliers and machine manufactures, in order to come to a right conclusion.
Apart from the actual cleaning procedure, it is also advisable to take into account the entire production process (including the type of processing as well as transport/storage containers for cleaners).
We offer companies our free consultancy service “Choosing the Right Cleaning Agent” - with no strings attached! If you're interested, send us your contact details. Our team of cleaning experts will get in touch with you shortly.
SAFECHEM Europe GmbH
Tersteegenstr. 25
40474 Duesseldorf
Germany
service@safechem.com
Phone: +49 211 4389-300
Fax: +49 211 4389-389