Time constraints are a well-known production challenge. Because metal cleaning can be required multiple times in a production process, the cleaning capacity must be thoroughly aligned to prevent metal cleaning being the bottleneck in your production. Our experts are always happy to help you with competent advice about which methods could serve you best.
Industrial Parts Cleaning – what you need to know
What factors define successful industrial parts cleaning? What do cleaning chemicals actually do? What are the effects of modern cleaning equipment and how can safety systems for transportation, storage and handling of solvents or offerings like Chemical Leasing help me be more efficient?
Find the answers to these questions right here.
Industrial Parts Cleaning –
one definition,many requirements
Industrial parts cleaning is defined as the removal of unwanted contaminants such as oil, dust or dirt from workpieces. Sounds simple at first, but to achieve successful cleaning results, a lot of requirements have to be met. These requirements include:
» SAFE HANDLING
to properly manage risks.
» ENVIRONMENTAL COMPLIANCE
to produce in a sustainable way.
» HIGH REQUIREMENTS ON CLEANLINESS
to avoid quality issues (also in following production steps).
» HIGH THROUGHPUT
to meet your production goals.
» LEGAL COMPLIANCE
to be in line with relevant regulations.
The 4 Factors of successful
industrial parts cleaning
To meet all these requirements and to achieve the results you want from your metal cleaning, four factors are critical.

Time

Temperature
As a rule of thumb, the higher the temperature, the better the cleaning. However, the type of metal to be cleaned and the physical and chemical properties of the cleaning media will limit the maximum temperature.

Mechanics
Modern cleaning equipment can incorporate a variety of mechanical actions such as spraying, basket rotation, ultrasonic or combinations of these. The mechanical actions are adjusted to the cleaning requirement to optimize the process.

Chemistry
The type of metal to be cleaned and the physical and chemical properties of the contaminants to be removed are just some of the many parameters that need to be considered when choosing the right chemicals. The cleaning chemical you choose can have a major impact on production time, efficiency and financial viability.
How do cleaning agents
take effect?
Have you ever wondered how cleaning agents actually clean? Or why the best solution really depends on your individual process? Here are some of the reasons.

Solubility is one of the basic chemical effects responsible for cleaning. When the contaminant and the cleaning media have enough affinity, the soil will dissolve in the cleaning media, and can therefore be removed from the part.
Affinity can usually be roughly evaluated through an appreciation of polarity. A simple rule is to "clean like with like". Cleaning media will dissolve soil of similar polarity. Polarity is a complex chemical property directly linked to the formula of a chemical.
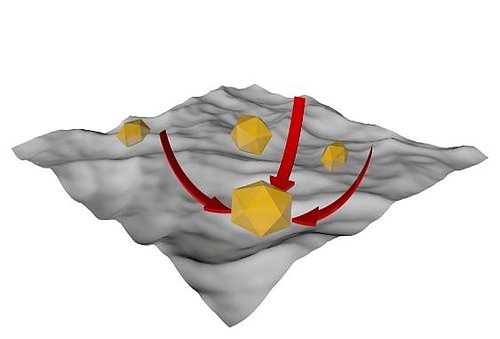
Density and viscosity are physicochemical properties. They describe the ability of the cleaning media to penetrate below the contamination (viscosity) and to physically remove it from its support (density). This chemical effect has an important impact when mechanical treatment is applied: denser chemicals, for example, will conduct ultrasonic better.
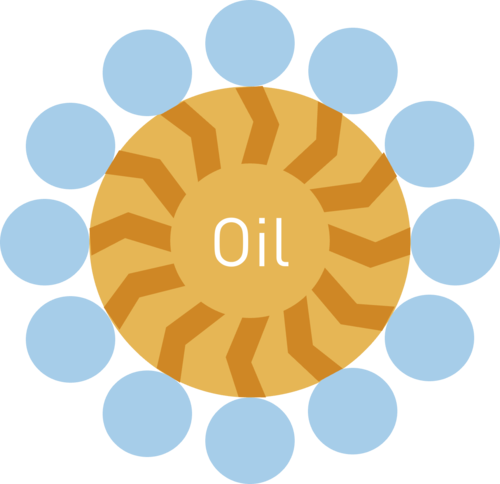
When the affinity of the solvent and the soil is not sufficient, the use of a surfactant is required. The surfactant usually presents two sides in its chemical formula: one side has affinity with the solvent, the other with the soil. It therefore enables you to remove soil that has no affinity with the cleaning media. However, the surfactant is consumed during the operation and new surfactant must be added, proportionate to the amount of soil removed. The composition of the cleaning media needs constant monitoring.
Legally compliant and
eco-friendly solvent cleaning
with modern equipment
Solvent cleaning is often stamped with an over-simplified "bad" in people's overall perception. However, its efficiency (time, resource efficiency, etc.) is often superior to alternatives promoted as "more sustainable", and when used in modern equipment, the potential negative impacts can be avoided.
Emissions can be virtually eliminated by using closed equipment. And as an added benefit, this system can be used for a large spectrum of cleaning applications, from the removal of oils and pastes to light contamination.
Closed cleaning technology with internal solvent recovery reduces the amount of waste you have to recycle, lowers your overall cleaning costs, and keeps you in line with all relevant regulations. And best of all, you can achieve all this without compromising on quality.
This is how modern
cleaning equipment works
Modern equipment can be used to clean a vast diversity of parts, from small parts to pipes up to several metres long. It can usually operate a number of different cleaning programmes that are tailored for the application.
The machines are equipped with a side or top entry/exit, together with protected loading/unloading zones. Additional technical features ensure low emissions and increased efficiency: the abatement loop, for example, reduces the solvent concentration in the cleaning chamber before unlocking the unloading door.
A TYPICAL CLEANING PROCESS
- PRE-WASHING
The cleaning chamber is flooded with solvent from tank number one. - EVACUATION TO TANK NUMBER 1
The solvent is transferred from the cleaning chamber back to tank number one. - IMMERSION/SPRAYING
The parts are cleaned by liquid solvent from tank number 2 (cleaner solvent tank), which is usually sprayed into the cleaning chamber. Cleaning power is improved by the use of ultrasonic (optional).
- EVACUATION TO DISTILLATION CHAMBER
The solvent is transferred from the cleaning chamber to the distillation unit. - VAPOUR CLEANING
Pure solvent vapour generated by the distillation unit is sent to the cleaning chamber and condenses on the cooler parts. The residual oil film is then removed. This step usually allows a very high precision cleaning. - VACUUM DRYING
By applying a vacuum to the cleaning chamber, the evaporation of the solvent is accelerated. - VENTILATION
The cleaning chamber is ventilated until normal atmospheric conditions are achieved. During the process, the solvent concentration in the cleaning chamber is controlled, and the door opens only if the concentration is below the values specified by the Industrial Emissions Directive (IED).As an option, the equipment can be operated under vacuum, thus lowering the distillation temperatures and allowing permanent control of the vapour emissions from the cleaning machine.
A closed cleaning system also protects your competitiveness
Many studies have shown the effectiveness and savings achieved by utilizing a closed cleaning system. Examples show that modern cleaning equipment combined with innovative service offerings like Chemical Leasing can reduce solvent consumption by up to 99.5%. This reduces overall costs while providing high-quality results and therefore maintains competitiveness.